
About zalunfiban
Zalunfiban is an investigational next-generation GPIIb/IIIa platelet inhibitor designed for rapid pre-hospital treatment of STEMI (ST-segment elevation) heart attacks.
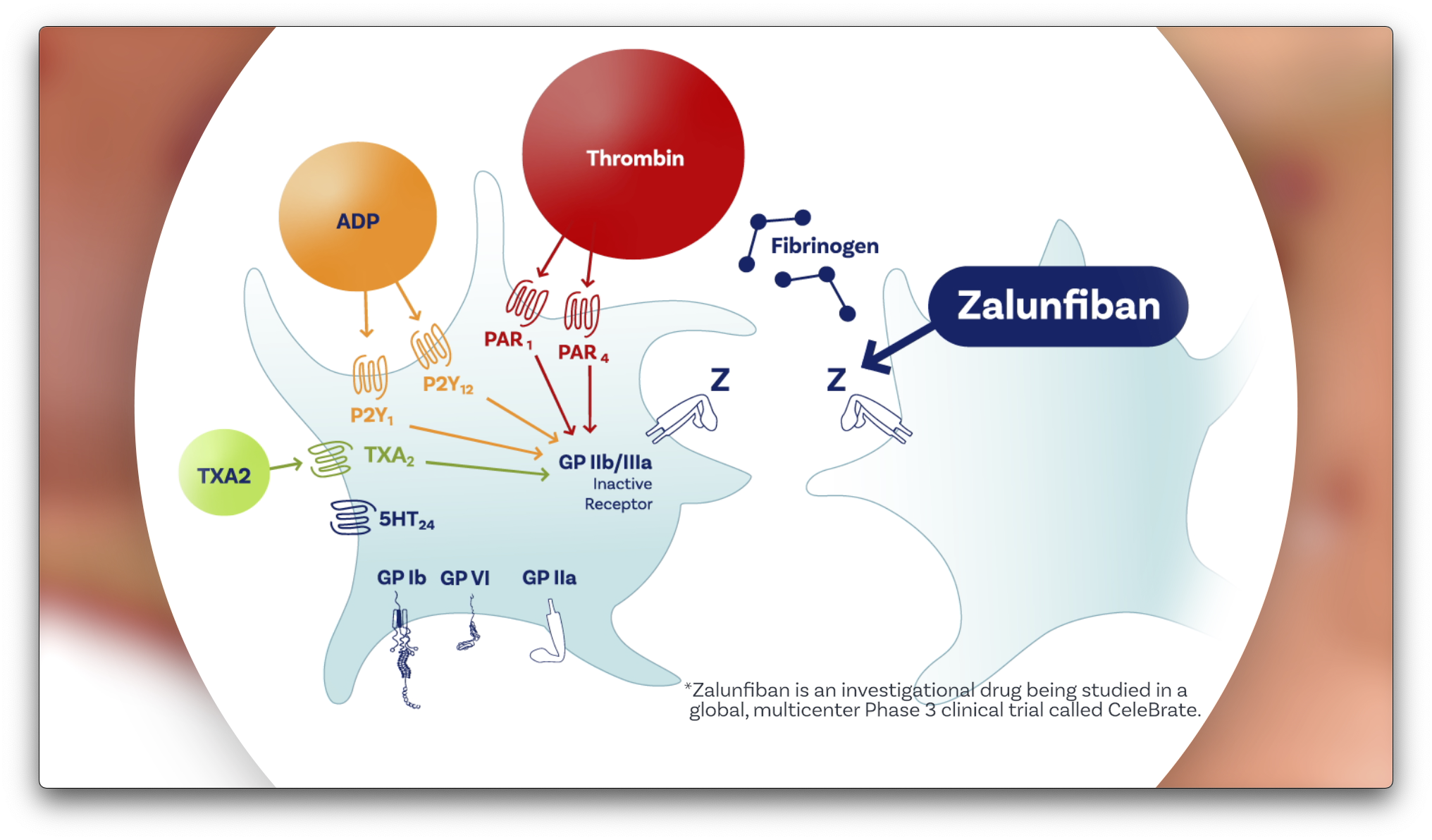



Zalunfiban mechanism of action
Most U.S. STEMI patients aren’t treated in time to meet AHA/ACC guidelines for effective treatment. 1Jollis et al. JAMA 2022;328;(20):2033-2040. doi:10.1001/jama.2022.20149
While in-hospital management of heart attacks has greatly improved over the years, treatment at the scene of a heart attack and during transport has stagnated.
The extent of irreversible heart-muscle damage increases with every minute the blood vessel remains closed. This damage can later result in heart failure, one of the most common causes of hospitalization and death in the U.S.
Zalunfiban is designed to treat STEMI heart attacks—the most severe form of heart attack, where blood flow to a portion of the heart is almost always cut off by a blood clot. About 40% of heart attacks are STEMIs.
2 Dudas et al, Trends in Out-of-Hospital Deaths Due to Coronary Heart Disease in Sweden (1991 to 2006). Circulation. 2011;123:46-52
3 [1] Bentur OS, et al. Assessing the Pharmacodynamics of RUC-4 (zalunfiban), a Novel αIIbβ3 Antagonist, Using VerifyNow Assays in Patients with ST-Segment Elevation Myocardial Infarction (STEMI) Treated with Aspirin and Ticagrelor [abstract]. Res Pract Thromb Haemost. 2021; 5 (Suppl 1).
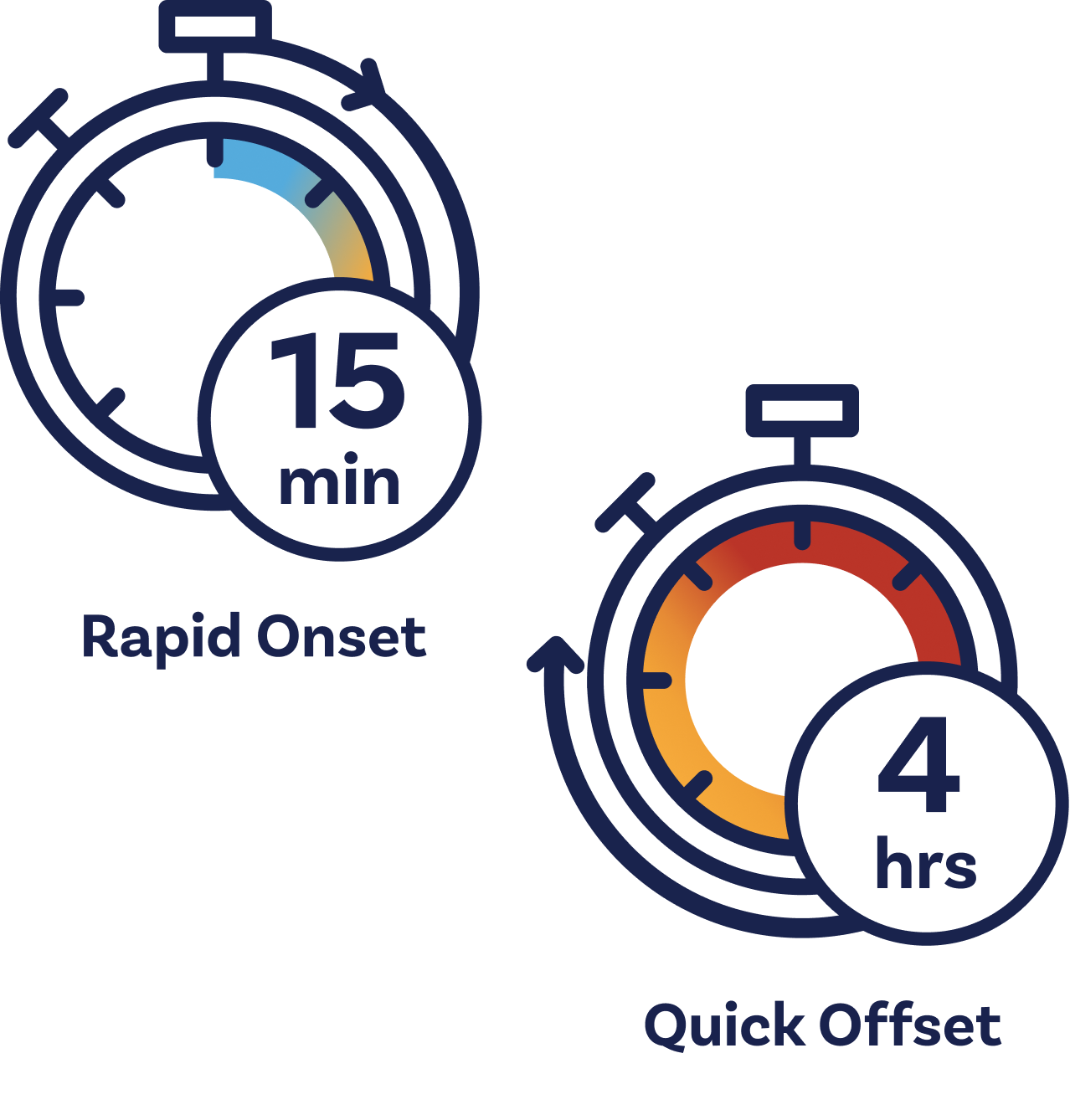
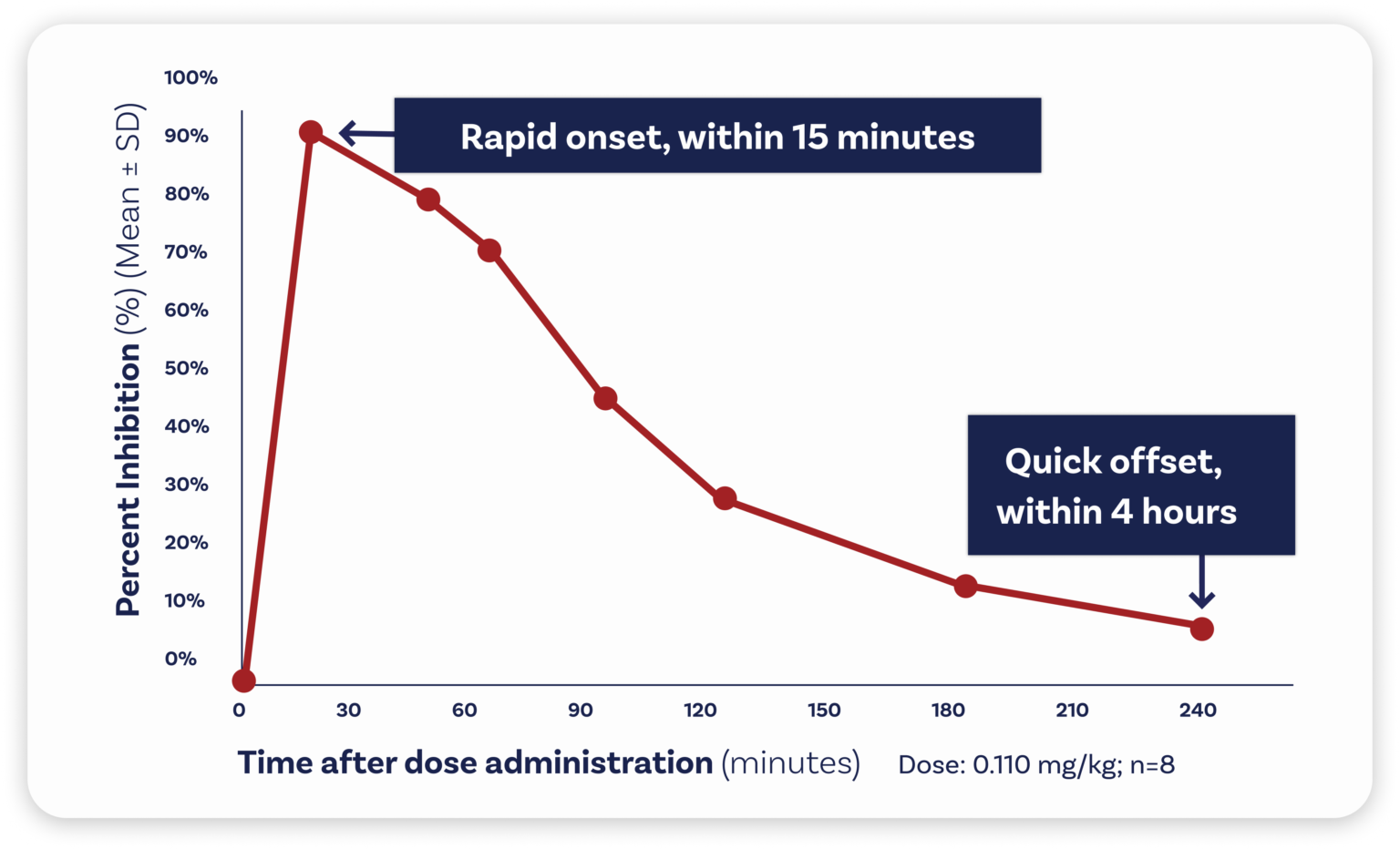
Zalunfiban acts within 15 minutes.
Zalunfiban is a next-generation investigational GPIIb/IIIa inhibitor that was specifically designed for medical first responders and emergency department staff to administer by subcutaneous injection, allowing a full dose to be contained in a volume of less than 1 milliliter (less than ¼ teaspoon). It reaches maximal effect within 15 minutes and its antiplatelet effect wears off in about two hours and is back to baseline within 4 hours.4Bentur OS, et al. Assessing the Pharmacodynamics of RUC-4 (zalunfiban), a Novel αIIbβ3 Antagonist, Using VerifyNow Assays in Patients with ST-Segment Elevation Myocardial Infarction (STEMI) Treated with Aspirin and Ticagrelor [abstract]. Res Pract Thromb Haemost. 2021; 5 (Suppl 1).
It also was designed to minimize the risk of thrombocytopenia, a rare but serious side effect of other GPIIb/IIIa inhibitors.
Bor WL, et. al. Pharmacokinetics, pharmacodynamics, and tolerability of subcutaneous administration of a novel glycoprotein IIb/IIIa inhibitor, RUC-4, in patients with ST-segment elevation myocardial infarction. EuroIntervention 2021;17-online publish-ahead-of-print May 2021
Zalunfiban is an investigational agent; it has not been approved for any use and its safety and efficacy have not been established.

Zalunfiban was designed to be prompt, potent and predictable.
Prompt
- It can easily be given subcutaneously and begins to enter the bloodstream immediately, reaching its maximal effect within 15 minutes. Its rapid action is especially important when transport to the hospital is lengthy or delayed.
Potent
- While commonly used platelet inhibitors only block specific activators, zalunfiban blocks platelet aggregation induced by all platelet activators, including thrombin, thromboxane and ADP.5Orzalkiewicz M, et al. Comparison of Routine Versus Selective Glycoprotein IIb/IIIa Inhibitors Usage in Primary Percutaneous Coronary Intervention (from the British Cardiovascular Interventional Society). American Journal of Cardiology. 124(2019) P373-380.
- A small volume of drug administered subcutaneously can be rapidly absorbed.
Predictable
- Zalunfiban reaches maximal effect within 15 minutes in virtually all patients, while its antiplatelet effects also rapidly diminish (in less than two hours),6Bentur OS, et al. Assessing the Pharmacodynamics of RUC-4 (Zalunfiban), a Novel αIIbβ3 Antagonist, Using VerifyNow Assays in Patients with ST-Segment Elevation Myocardial Infarction (STEMI) Treated with Aspirin and Ticagrelor [abstract]. Res Pract Thromb Haemost. 2021; 5 (Suppl 1). which may reduce the risk of bleeding and allow for rapid cardiac surgery if needed.



Zalunfiban's potential impact
STEMI patients
There is no antiplatelet treatment routinely used for heart attacks in the U.S. in the pre-hospital setting other than aspirin.

First responders
Zalunfiban is designed for use by medical first responders via a subcutaneous injection, reaching maximal effect within 15 minutes.
Interventional cardiologists
Zalunfiban’s antiplatelet effect wears off in about two hours, which may allow for rapid cardiac surgery or PCI if needed.
*Studied in the CeleBrate trial.
Competitive Landscape
|
Aspirin |
Ticagrelor (Brilinta®) Prasugrel (Effient®) |
Tirofiban (Aggrastat®) Eptifibatide (Integrilin®) |
Cangrelor (Kengreal®) |
Selatogrel |
Zalunfiban (Disaggpro™) |
|
|---|---|---|---|---|---|---|
|
Rapid onset of action |
 |
 |
 |
 |
||
|
Easy to administer in pre-hospital setting |
 |
 |
 |
 |
||
|
Inhibits all platelet aggregation pathways |
 |
 |
||||
|
Can open a closed coronary artery |
 |
 * * |
||||
|
Predictable response |
 |
 |
 |
 |
||
|
Absorption independent of opioid administration |
 |
 |
 |
|||
|
Low risk of thrombocytopenia |
 |
 |
 |
 |
 * * |
*Studied in the CeleBrate trial.
Swipe or tap arrow to see more comparisons







